FAQs on Section 1135 Waivers: What Health Care Providers Should Know
This Bulletin is brought to you by AHLA’s In-House Counsel Practice Group and the Public Health System Affinity Group of the Hospitals and Health Systems Practice Group.
- March 18, 2020
- Sarah Swank , Nixon Peabody LLP
- Harsh P. Parikh , Nixon Peabody
- Joanna Cohen , Nixon Peabody LLP
The health care industry is finally getting some much-needed flexibility to maneuver through the COVID-19 coronavirus pandemic. Following a joint letter by the American Hospital Association, the American Medical Association, and the American Nursing Association, on Friday, March 13, 2020, President Donald J. Trump issued a Proclamation on Declaring a National Emergency Concerning the Novel Coronavirus Disease (COVID-19) Outbreak under the National Emergencies Act. The President also directed hospitals and medical facilities throughout the country to assess their disaster preparedness posture and prepare to surge capacity and capabilities.
The Emergency Proclamation opens the door for the U.S. Department of Health and Human Services (HHS) to offer health care providers relief through waivers under Section 1135 of the Social Security Act (Section 1135). Below, we provide responses to key questions on Section 1135 waivers from providers that are on the frontlines of this global pandemic.
Why was the emergency proclamation necessary?
Health care providers around the country are bracing for an influx of new COVID-19 cases and looking for ways to expand their capacity to care for COVID-19 patients. Last week, hospitals started delaying elective surgeries, limiting visitations, enhancing their telehealth capabilities, and expanding their clinical staff to support a growing demand. These moves, however, continue to be hampered by existing federal rules and regulations, including the CMS conditions of participation (CoPs), Emergency Medical Treatment and Labor Act (EMTALA), and the Health Insurance Portability and Accountability Act (HIPAA). The presidential proclamation allows HHS, through Section 1135 waivers, to enhance flexibilities that will support an expected surge of infected patients.
What are Section 1135 waivers?
Section 1135 waivers or modifications allow health care providers to be exempt (or waived) from certain statutory requirement of the Social Security Act and its implementing regulations. As part of the government’s initial response to the growing pandemic, the bi-partisan Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020 appropriated $500M to telehealth reimbursement and allowed HHS to issue Section 1135 waivers to remove traditional limitations for virtual care.
CMS will implement these flexibilities as necessary and appropriate to accommodate the needs of those impacted by the novel coronavirus outbreak. Importantly, waiver or modification granted as a result of the COVID-19 emergency may be applied retroactively starting March 1, 2020.
Which “blanket” waivers have been granted?
Following the National Emergency Proclamation, HHS’ Centers for Medicare & Medicaid Services (CMS) issued several nationwide blanket waivers for health care providers that CMS Administrator Seema Verma described as “a godsend for those on the frontlines of the fight against this new virus.” CMS issued several blanket waivers, which are immediately available to all health care providers. The following blanket waivers are available for the duration of the national emergency:
- Skilled Nursing Facility (SNF) Coverage. Exercising its authority under Section 1812(f) of the Social Security Act, CMS is waiving the so-called SNF three-day rule. Medicare beneficiaries can now be transferred to an SNF without a three-day prior inpatient hospitalization. Beneficiaries that recently exhausted their SNF benefits can also obtain renewed coverage. In addition, CMS is waiving certain timeframe requirements for data submissions for SNFs.
- Critical Access Hospitals (CAHs). CMS is waiving requirements to allow CAHs to have in excess of 25 beds and to allow patients of CAHs to stay longer than 96 hours.
- Long-Term Care Hospitals (LTCHs). CMS is waiving requirements to allow LTCHs to exclude patient stays where an LTCH admits or discharges patients in order to meet the demands of the emergency from the 25-day average length of stay requirement.
- Housing Acute Care Patients in Step-Down Beds. CMS is waiving requirements to allow acute care hospitals to house acute care inpatients in excluded distinct part units that are appropriate for acute care inpatients and continue to be reimbursed through Medicare’s Inpatient Prospective Payment System.
- Durable Medical Equipment Prosthetics, Orthotics, and Supplies (DMEPOS). If any DMEPOS is lost, destroyed, irreparably damaged, or otherwise rendered unusable or unavailable as a result of the emergency, CMS contractors may waive replacement requirements such that beneficiaries may access DMEPOS without a face-to-face meeting, a new physician’s order, or new medical necessity documentation. Suppliers must still include a narrative description on the claim explaining why the DMEPOS must be replaced and maintain documentation on the need for the replacement.
- Relocating Inpatient Rehabilitation and Psych Unit Patients in Acute Care Unit of Hospitals. CMS is waiving requirements to permit acute care hospitals with distinct part inpatient psychiatric units and/or inpatient rehabilitation units to relocate inpatients to an acute care bed, if necessary, as a result of the emergency. Additionally, CMS is allowing certain exclusions from calculations that impact a facility’s classification as an Inpatient Rehabilitation Facility under the so-called 60% rule.
- Home Health Agencies. CMS is providing relief to home health agencies on the timeframes related to OASIS transmission. In addition, CMS is allowing Medicare administrative contractors to extend the auto-cancellation date of Requests for Anticipated Payment during emergencies.
- Provider Locations. CMS is waiving requirements that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state.
- Enrollment in Medicare. CMS is allowing new non-certified Part B suppliers, physicians, and non-physician practitioners to expeditiously obtain temporary Medicare billing privileges. CMS is also waiving certain application fees, criminal background checks, and site visits with respect to provider enrollment.
- Medicare Administrative Appeals. CMS is providing additional flexibilities pertaining to Medicare appeals in fee-for-service, Medicare Advantage, and Part D, including giving providers an extension to file an appeal and processing requests for appeals that do not meet the required elements using the information available.
In addition, effective March 15, 2020, HHS will waive sanctions and penalties against a covered hospital that does not comply with the following provisions of the HIPAA Privacy Rule:
- the requirements to obtain a patient’s agreement to speak with family members or friends involved in the patient’s care
- the requirement to honor a request to opt out of the facility directory.
- the requirement to distribute a notice of privacy practices.
- the patient’s right to request privacy restrictions.
- the patient’s right to request confidential communications.
Finally, effective March 6, 2020, CMS will be waiving existing limitations on Medicare coverage for telehealth during the COVID-19 emergency. These visits will be considered the same as in-person visits and paid at the same rate as regular, in-person visits. Under the broad waiver, Medicare telehealth services may now be furnished to beneficiaries in any health care facility and in their home. The following chart from CMS summarizes the types of telehealth visits that will be available during the duration of the COVID-19 emergency:
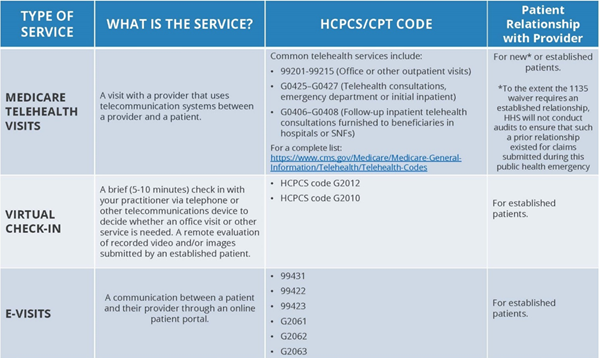
These blanket emergency waivers do not require submission of individualized requests by providers. Instead, providers should review their operations to determine how the increased flexibilities facilitated by these waivers can better support their operations and caring for COVID-19 cases in their communities.
What about case-by-case waivers?
The national emergency declaration permits the federal agency to waive other requirements related to Medicare, Medicaid, and the Children’s Health Insurance Programs (CHIP). These waivers are done on a case-by-case basis. Health care providers must assess their needs and identify any federal laws, the waiver of which would facilitate serving patients in their communities during the COVID-19 pandemic. For example, providers should evaluate whether to seek specific waivers of any of the following federal laws:
- Other CoPs. Waivers pertaining to conditions of participation (CoPs) and conditions of payments for Medicare and Medicaid claims.
- Emergency Medical Treatment and Labor Act (EMTALA). Modifications of activities under EMTALA that would allow (1) the transfer of unstabilized patients other than in accordance with the EMTALA regulations and (2) the direction or relocation of patients to receive medical screening at an alternative off-campus location.
- Stark Law. Sanctions for violations of the Stark Law, such as for physician staffing without a written agreement, as required by many Stark Law exceptions.
- Timing. Modifications of existing deadlines and timetables to perform required activities.
- Medicare Advantage. Limitations on the ability to make direct payments to providers for services to Medicare Advantage enrollees.
Although many of the blanket waivers will prove to be useful, most health care providers will likely need to submit a specific waiver request to take advantage of the full scope of flexibilities available under Section 1135.
How do providers obtain Section 1135 Waivers?
Health care providers must submit requests to operate under Section 1135 authority to both their State Survey Agency (and/or the applicable accreditation organization) and their CMS Regional Office. The waiver request should include key information about the enrolled facility and a brief justification for requesting the waiver or modification. Health care providers do not need to submit waiver information to take advantage of the blanket waivers. Waiver requests should be made in consultation with legal counsel.
What other requirements should providers be mindful of?
Although Section 1135 waivers can provide key tools to address surges in demand, health care providers must also be mindful of state licensure laws and local requirements. Many state governors have already proclaimed state-wide emergencies, triggering similar flexibilities under state law. Health care providers and suppliers located in areas with the COVID-19 outbreak should also consider certain disaster assistance for capital expenditures.